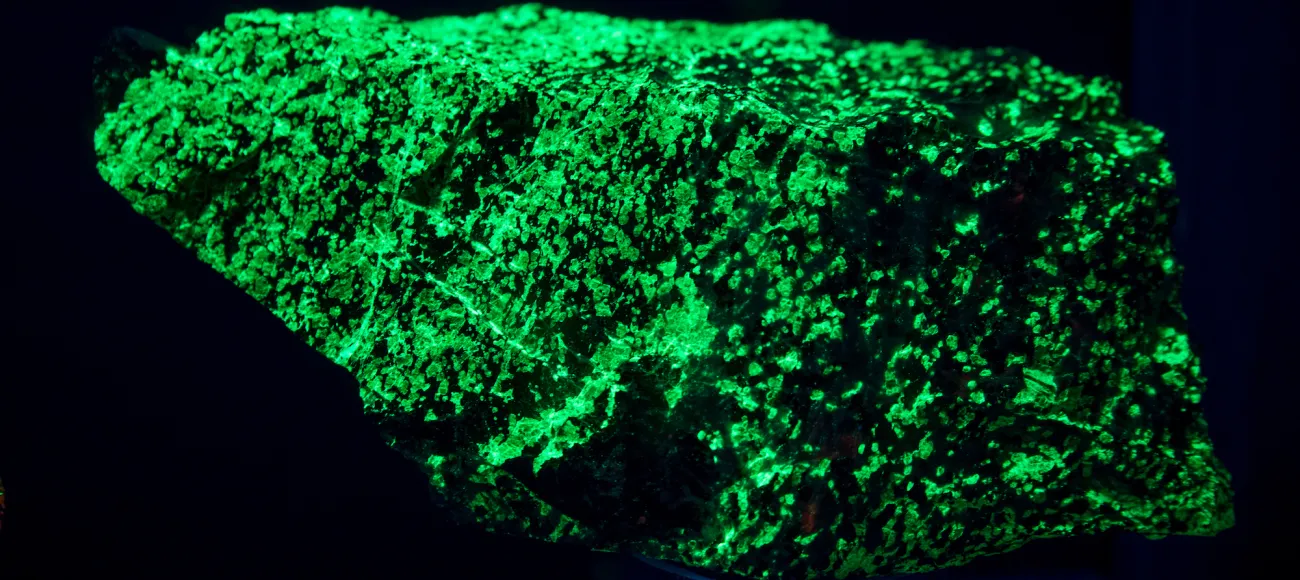
Why do rocks glow?
Inside a small room in Rudolph Hall are shelves of dull, brown rocks that begin to glow in vivid greens, oranges, blues and purples when a button is pushed and ultraviolet lights turn on.
Many of the rocks on display were collected by Jill Pasteris, PhD, professor of earth and planetary sciences in Arts & Sciences, although she says it is really her colleague Bob Osburn we should thank for the display, because he made sure it was put into the blueprints for the building, hand-selected the samples, and helped design the lighting system.
For a geologist, fluorescence is a fascinating riddle. Only about 15 percent of minerals fluoresce and not every specimen of a mineral that can fluoresce does so. Typically fluorescence occurs when a mineral contains impurities known as “activators,” such as a light salting of manganese. Different activators can make the same mineral fluoresce in different colors. In addition to activators, there are quenchers, impurities that prevent the mineral from fluorescing. An admixture of iron, for example, can quench fluorescence.
What’s marvelous about all this is there’s absolutely no practical reason why rocks should glow. In this way the fluorescing rocks resemble the twinkling stars; their beauty brings people up short and, for a moment, spectacularly interrupts their routine preoccupations.
On display...